Hearing, one of our primary senses, is essential for daily communication. Yet it has remained a mystery for forty years the molecular identity of the auditory transduction ion channel that are at the heart of human sound perception.
Recently, the mystery has been unveiled by the joint research of teams led by Prof. Yan Zhiqiang (School of Life Sciences, Fudan University), Prof. Motoyuki Hattori (School of Life Sciences, Fudan University) and Prof. Osamu Nureki (Tokyo University). The study was published in Neuron under the title “TMC1 and TMC2 proteins 1 are pore-forming subunits of 2 mechanosensitive ion channels” on November 21, which proves that the transmembrane channel-like (TMC) 1 and 2 genes are the auditory transduction ion channels in the cochlea hair cells.

Yan Zhiqiang
Looking into Auditory Transduction Ion channels
What are the auditory transduction ion channels in the cochlea hair cells? The researchers have managed to give an answer——TMC1/2.
According to Yan, auditory transduction may be damaged by multiple candidate genes in the auditory transduction channel, including TMC1 and TMC2, which are first identified in deaf human patients. Localized in stereocilia tips and expressed in hair cells, TMC1/2 are necessary for the mechanotransduction currents of hair cells. Previous research has proved that the transmembrane TMC1/2 are essential for hearing in mice from human genetic approaches.
“TMC1 mutations can alter the mechanosensitive currents in mice. However, whether TMC1 and TMC2 proteins are ion channels and whether TMC1 and TMC2 can be gated by mechanical force remain unclear,” Yan explains, “Ion channels serve the passive transmembrane transportation of inorganic ions, which allow the passage of ions from one side of the membrane where the concentration of ions is higher to the other. Ion channels are gated, which determines when and what ions can pass through.”
Previous studies reveal that TMC proteins are poorly trafficked to the cell membrane when expressed in cultured cells, hindering electrophysiological recording of TMC. To overcome the technical difficulty, researchers functionally reconstitute purified TMC1 and TMC2 proteins into liposomes to evaluate whether TMC proteins are indeed ion channels.
Yan says, “By liposome reconstitution, we generate liposomes that sharethe similar structure of bilayers with a real cell.”
To identify suitable candidate TMC1 and TMC2 proteins for purification, the researchers employ ortholog screening with fluorescence detection size-exclusion chromatography (FSEC) and FSEC-based thermostability (FSEC-TS) assays. After screening TMC proteins from 21 species, the researchers find that TMC1 from the green sea turtle Chelonia mydas (CmTMC1) and TMC2 from the budgerigar Melopsittacus undulatus (MuTMC2) show a high level of expression in insect cells.
Protein expression, Yan adds, is the process where information from a gene is used in the synthesis of a functional gene product, including genetic transcription and translation. Proteins are expressed at different levels under different circumstances. The researchers look for a higher level of expression and purification, just like farmers expecting to see a high yield of wheat.
To examine whether reconstituted TMC proteins are directly gated by mechanical stimuli, the researchers apply negative pressure through the patch-clamp recording pipette with a high-speed pressure clamp (HSPC) to stretch the membrane. It turns out that TMC proteins do exhibit mechanically gated channel activity and a progressive increase in current and the single-channel open probability of MuTMC2 upon pressure application. In addition, the researchers employ deafness-related TMC1 mutants to generate CmTMC1 mutants, which exhibit reduced/no channel activity or mechanosensitivity.
Yan expounds that although the current study focuses primarily on CmTMC1 and MuTMC2, the strong evolutionary conservation of CmTMC1 and MuTMC2 with the TMC1 and TMC2 of mice, highly similar to those of humans, suggests that mammalian TMC1 and TMC2 are also likely to be ion channels and to be mechanosensitive. What’s more, TCM1/2 is closely linked to human hearing loss.

Yan Zhiqiang with his students
Unblocking the Bottleneck in Auditory Research
“The key to understanding the auditory mechanism is to find out how mechanical signals of sound are converted into electrical signals. Yet scientists have been stuck there for 40 years,” Yan says, “Nothing more can be done before we unblock the bottleneck.”
In humans, the perception of sound begins with the organ of Corti in the inner ear, which harbors more than 16,000 hair cells. Mechanotransduction channels that convert the mechanical signals of sound into electrical signals are thought to be localized in these hair bundles, according to the research of Corey and Hudspeth 40 years ago. Yet the molecular identity of the auditory transduction ion channel has remained unknown.
Of all five of our senses — vision, taste, olfaction, touch, and hearing — the latter is the least well understood in terms of the molecular transduction mechanism. The researchers set out to unveil the mystery in 2017. With protein purification, functional reconstitution, and liposome recording, they finally shed light on auditory transduction ion channels, unraveling the mystery of hearing.
“Our research is expected to inspire clinical treatment for hearing loss, which in return will help researchers make further discoveries. This is a reciprocal process,” says Yan whose team will also work on the prevention, diagnosis and treatment of hereditary hearing loss and deafness in new-born babies.
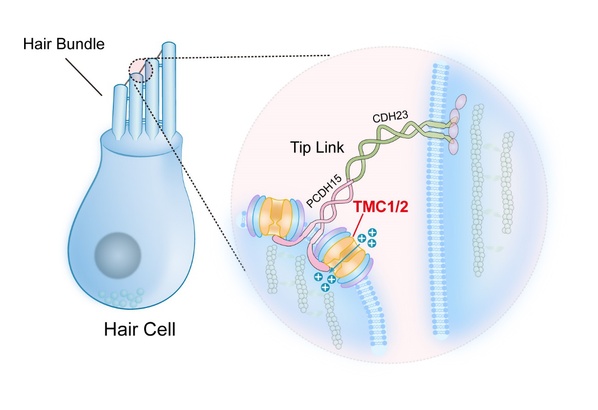
TMC1/2 in the auditory transduction
Yan, the corresponding author and lead contact of the research, conceived the project and designed he experiments. Prof. Motoyuki Hattori from the School of Life Sciences of Fudan University and Prof. Osamu Nureki from Tokyo University are the co-corresponding authors. Dr. Jia Yanyan from Yan’s team, postdoctoral researcher Zhao Yimeng from Hattori’s team, and Dr. Tsukasa Kusakizako are the co-first authors. Ph.D candidates Wang Yao, Pan Chengfang and Zhang Yuwei also made contribution for the research.