Recently, Shi Dongyun’s research team at the School of Basic Medical Sciences of Fudan University, has designed and developed a new formula of microcompartmental liver-targeted liposome encapsulated drug which can regulate redox balance in vivo. It has successfully regulated redox balance of microenvironment in diabetic animals, effectively normalizing peripheral blood glucose level and preventing type 2 diabetes. Their paper, titled "Liver-targeted Nano-MitoPBN normalizes glucose metabolism by improving mitochondrial redox balance", was published in Biomaterials.
(https://doi.org/10.1016/j.biomaterials.2019.119457)
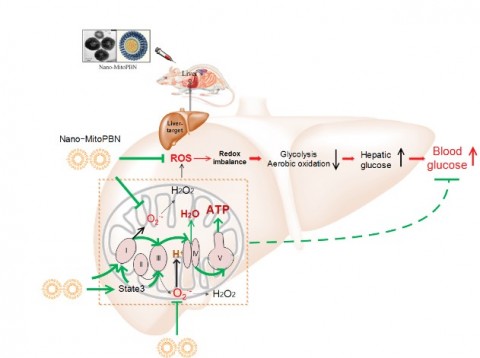
To maintain redox balance has been considered as the basis of human health, since redox imbalance has been implicated in many diseases including tumors, cardiovascular and metabolic diseases. At the 654th Xiangshan Scientific Conference themed Redox balance and new strategies for diagnosis, prevention and treatment of major diseases, wider application of redox balance theories to prevention and treatment of major diseases in order to establish a new diagnosis and treatment system was called for by many experts. In response to this appeal, Shi's team took the lead in making breakthroughs in diabetes prevention and treatment.
Redox imbalance in liver microcompartment has been implicated in type 2 diabetes mellitus which is characterized by abnormal glucose metabolism and hyperglycemia. Shi’s group used liposome encapsulated MitoPBN to target liver mitochondria. Because of microcompartmentalization, nano-MitoPBN is able to target hepatic mitochondria specifically to scavenge superoxide/hydrogen peroxide generated from mono-electron leak of electron transport chain (ETC) complex I and III, thereby regulating and maintaining the dynamic mitochondrial redox balance. Nano-MitoPBN increased mitochondrial state 3 respiratory rate and respiratory control ratio (RCR), resulting in decreased NADH:NAD+ ratio, improved mitochondrial oxidative energy coupling and ATP synthesis, thus alleviating ROS induced-mitochondrial dysfunction. The functional mitochondria promoted the substrate oxidation by the liver, resulting in increased glycolysis and TCA cycle, which directly enhances glucose decomposition, thus decreasing the peripheral blood glucose level and improving the impaired glucose tolerance in diabetic animals. Their study suggests the potential of using antioxidative nanomedicines that target liver mitochondria for diabetes mellitus.
Shi’s team has provided a novel drug design for diabetes mellitus by using a new formula of liposome nanoparticles containing cationic groups to achieve the precise intervention in the liver microcompartmental environment. By regulating the imbalanced redox state in liver microcompartment, the abnormal liver mitochondrial bioenergetic function and glucose metabolism can be restored, resulting in normalization of peripheral blood glucose level and improvement of diabetes mellitus.
Their results provide not only a new idea for the development of novel anti-diabetic drugs, but also a foundation for clinical practice of precise intervention for redox balance.





